The complete question is as follows: I want to make a homemade cake recipe you need 100 grams of sugar, calculate the moles to which it is equivalent and the number of molecules of sucrose.
Answer: In 100 grams of sugar there are 0.292 moles and the number of molecules of sucrose are
 .
.
Step-by-step explanation:
Given: Mass = 100 g
Molar mass of sugar = 342.3 g/mol
As number of moles is the mass of substance divided by its molar mass.
Hence, moles of sucrose are calculated as follows.
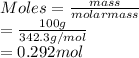
According to mole concept, 1 mole of every substance contains
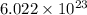 molecules.
molecules.
Hence, the number of molecules present in 0.292 mol are calculated as follows.
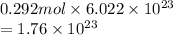
Thus, we can conclude that in 100 grams of sugar there are 0.292 moles and the number of molecules of sucrose are
 .
.