Answer:
1-chlorobutane
Step-by-step explanation:
Given that :
-- The compound A is made to react with alcoholic KOH and produce compound B.
-- The compound B on Ozonolysis produces methanol as well as propanol
The compound A should be a haloalkanes as treatment of haloalkanes with the alcoholic KOH, it will give alkene.
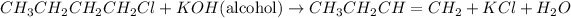
So, the compound B should be Butene
Now the ozonolysis of the compound B or butene will give methanol and propanal as shown :
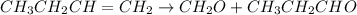
Therefore, the compound A is 1-chlorobutane.