Answer: The enthalpy of the reaction is -1791.31 kJ.
Step-by-step explanation:
Enthalpy change is the difference between the enthalpies of products and the enthalpies of reactants each multiplied by its stoichiometric coefficients. It is represented by the symbol
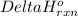
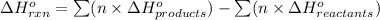 .....(1)
.....(1)
For the given chemical reaction:
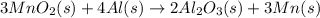
The expression for the enthalpy change of the reaction will be:
![\Delta H^o_(rxn)=[(2 * \Delta H^o_f_((Al_2O_3(s)))) + (3 * \Delta H^o_f_((Mn(s))))] - [(3 * \Delta H^o_f_((MnO_2(s)))) + (4 * \Delta H^o_f_((Al(s))))]](https://img.qammunity.org/2022/formulas/chemistry/high-school/y57lgiqlq1s27spffnyr8zewf8punagu1s.png)
Taking the standard heat of formation values:
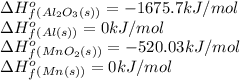
Plugging values in the above expression:
![\Delta H^o_(rxn)=[(2 * (-1675.7))+(3 * 0)] - [(3 * (-520.03))+(4 * 0)]\\\\\Delta H^o_(rxn)=-1791.31 kJ](https://img.qammunity.org/2022/formulas/chemistry/high-school/4lc3pm8oreevkf0ue240nafm5q2ni54tpw.png)
Hence, the enthalpy of the reaction is -1791.31 kJ.