Answer:
For a: The given salt is acidic in nature.
For b: The given salt is neutral in nature.
For c: The given salt is neutral in nature.
Step-by-step explanation:
Salts are formed by the combination of an acid and a base. There are 3 types of salt:
- Acidic salt: It is formed by the combination of strong acid and weak base.
- Basic salt: It is formed by the combination of a strong base and weak acid.
- Neutral salt: It is formed by the combination of strong acid and strong base or weak acid and weak base.
For the given options:
(a):
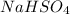
This salt is formed by the combination of sodium hydroxide (strong base) and the partial dissociation of sulfuric acid (strong acid). It also has a replaceable hydrogen ion which can be produced when dissolved in water. Thus, the given salt is acidic in nature.
(b):
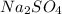
This salt is formed by the combination of sodium hydroxide (strong base) and sulfuric acid (strong acid).
Thus, the given salt is neutral in nature.
(c):
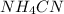
This salt is formed by the combination of ammonium hydroxide (weak base) and hydrocyanic acid (weak acid).
Thus, the given salt is neutral in nature.