Answer:
Given solutions are:
a) 0.1 M of H2S
b) 0.1 M of HNO2
c) 0.1 M of H2SO3
d) 0.1 M of NH4Cl
Step-by-step explanation:
The electrical conductivity of a solution is directly proportional to the number of ions present in it.
All the given solutions have the same concentration.
But on dissociation, each solution produces a different number of ions as shown below:
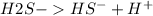
2 ions per molecule.
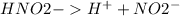
2 ions per molecule.
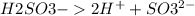
3 ions per molecule.
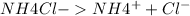
2 ions per molecule.
Answer:
0.1M H2SO3 has high electrical conductivity.