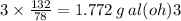Answer:
approximately 1.772 grams
Step-by-step explanation:
molecular mass of AlCl3 is 132 g per mole and of Al(OH)3 is 78 g per mole
the reaction is
AlCl3 + 3 NaOH ---> Al(OH)3 + 3 NaCl
from the reaction it is clear that 1 mole AlCl3 makes 1 mole Al(OH)3
implies 132g AlCl3 gives 78g Al(OH)3
Implies 3g AlCl3 gives