Solution :
From the balanced chemical equation, we can say that 1 moles of KBr will produce 1 moles of KCl .
Moles of KBr in 102 g of potassium bromide.
n = 102/119.002
n = 0.86 mole.
So, number of miles of KCl produced are also 0.86 mole.
Mass of KCl produced :
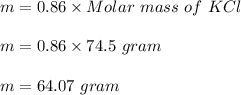
Hence, this is the required solution.