Answer:
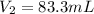
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the volume of the new solution by using the general formula of dilution:
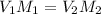
In such a way, we solve for the final volume, V2, to obtain:
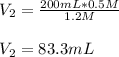
Regards!