Answer:
A) 3.6 x 10^-5.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly necessary to write the equilibrium expression for the dissociation of strontium chromate:
![Ksp=[Sr^(2+)][CrO_4^(2-)]](https://img.qammunity.org/2022/formulas/chemistry/college/xxp5q7ip868thg9fqb9m586avg088fw8eh.png)
Thus, since strontium and chromate ions are in a 1:1 mole ratio, we can tell the concentration of both ions as the same; and therefore, the Ksp is:
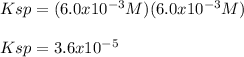
Or option A) 3.6 x 10^-5.
Regards!