Answer:
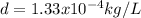
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the required density by knowing it is calculated by dividing mass over volume:
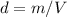
Therefore, we plug in the given kilograms and liters to obtain:
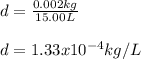
Which shows the proper scientific notation.
Regards!