Answer: The number of cations are
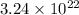 and number of anions are
and number of anions are
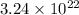 in 6.42 g of KBr.
in 6.42 g of KBr.
Step-by-step explanation:
The molar mass of KBr is (39.10 + 79.90) g/mol = 119.00 g/mol
Now, the dissociation equation for KBr is as follows.
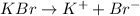
This means that 1 mole of KBr is forming 1 mole of
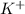 (cation) and 1 mole of
(cation) and 1 mole of
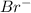 (anion).
(anion).
According to mole concept, 1 mole of every substance contains
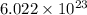 atoms. Hence, number of cations present in 6.42 g KBr is calculated as follows.
atoms. Hence, number of cations present in 6.42 g KBr is calculated as follows.
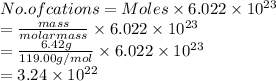
As according to the equation, there are equal number of moles of both cation and anions.
This means that the number of anions are also
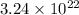 .
.
Thus, we can conclude that the number of cations are
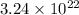 and number of anions are
and number of anions are
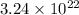 in 6.42 g of KBr.
in 6.42 g of KBr.