Answer:
Ka = 6.02x10⁻⁶
Step-by-step explanation:
The equilibrium that takes place is:
We calculate [H⁺] from the pH:
- [H⁺] =
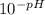
Keep in mind that [H⁺]=[A⁻].
As for [HA], we know the acid is 0.66% dissociated, in other words:
We calculate [HA]:
Finally we calculate the Ka:
- Ka =
![([9.12x10^(-4)]*[9.12x10^(-4)])/([0.138])](https://img.qammunity.org/2022/formulas/chemistry/college/rik1orrkmrf303xrowww4niqv2qa4ugmmh.png) = 6.02x10⁻⁶
= 6.02x10⁻⁶