Answer: Two compounds below that can be used to make a buffer solution are acetic acid and acetate ion.
Step-by-step explanation:
A solution that helps in resisting change in pH when a small amount of acid or base is added to it is called a buffer solution.
A buffer solution consists of a mixture of weak acid and its conjugate base.
Acetic acid has a chemical formula
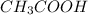 and its conjugate base is
and its conjugate base is
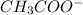 .
.
This base is also called acetate ion.
Acetic acid is a weak acid because it dissociates weakly when added to a solvent like water.
Therefore, acetic acid and acetate ion will form a buffer solution.
Thus, we can conclude that two compounds below that can be used to make a buffer solution are acetic acid and acetate ion.