Answer:
The binding energy is -882.5 MeV.
Step-by-step explanation:
The nuclear binding energy of Boron-9 can be found as follows:
![B = [Zm_(p) + Nm_(n) - M]c^(2)](https://img.qammunity.org/2022/formulas/physics/college/q5oyc49cjlhkodzfyjkdaqytxj70v4tlpk.png)
Where:
Z: is the number of protons = atomic number = 5
N: is the number of neutrons = A - Z = 9 - 5 = 4
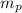 : is the mass of the proton = 1.0078251 u
: is the mass of the proton = 1.0078251 u
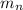 : is the mass of the neutron = 1.0086649 u
: is the mass of the neutron = 1.0086649 u
M: is the atomic mass of B-9 = 9.0133288 u
c² = 931.5 MeV/u
Hence, the nuclear binding energy is:
![B = [Zm_(p) + Nm_(n) - M]c^(2) = [4*1.0078251 u + 4*1.0086649 u - 9.0133288 u]*931.5 MeV/u = -882.5 MeV](https://img.qammunity.org/2022/formulas/physics/college/higkr1xalqdmcrx39wnch4ox1libeeny7k.png)
Therefore, the binding energy is -882.5 MeV.
I hope it helps you!