Answer: For the given reaction
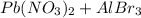 the products are
the products are
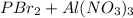 .
.
Step-by-step explanation:
A reaction in which two substances chemically combine together and results in the formation of a new substance is called a chemical reaction.
For example,
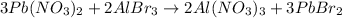
Products are the species that are written on the right side of an arrow in a chemical reaction equation.
Hence, we can conclude that for the given reaction
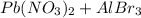 the products are
the products are
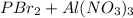 .
.