Answer:

Step-by-step explanation:
In this problem, volume and pressure are changing, so we use Boyle's Law. This states that the volume of a gas is inversely proportional to the pressure. The formula for this law is:
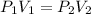
Originally the gas has a volume of 30 milliliters and a pressure of 4 atmospheres.
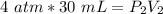
The pressure is changed to 2 atmospheres, but the new volume is unknown.
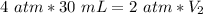
We want to solve for the new volume (V₂). It is being multiplied by 2 atmopsheres. The inverse operation of multiplication is division, so we divide both sides by 2 atm.
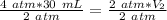
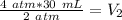
The units of atmospheres (atm) cancel.
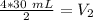
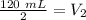
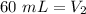
The new volume of the gas is 60 milliliters.