Answer:

Step-by-step explanation:
We are given a number of particles and asked to convert to moles.
1. Convert Particles to Moles
1 mole of any substance contains the same number of particles (atoms, molecules, formula units) : 6.022 *10²³ or Avogadro's Number. For this question, the particles are not specified.
So, we know that 1 mole of this substance contains 6.022 *10²³ particles. Let's set up a ratio.
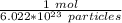
We are converting 2.98*10²³ particles to moles, so we multiply the ratio by that value.
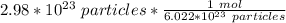
The units of particles cancel.
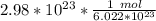
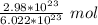
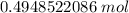
2. Round
The original measurement of particles (2.98*10²³) has 3 significant figures, so our answer must have the same.
For the number we found, 3 sig figs is the thousandth place.
The 8 in the ten-thousandth place (0.4948522086) tells us to round the 4 up to a 5 in the thousandth place.
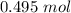
2.98*10²³ particles are equal to approximately 0.495 moles.