Answer: The final concentration of aluminum cation is 0.335 M.
Step-by-step explanation:
Given:
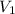 = 47.8 mL (1 mL = 0.001 L) = 0.0478 L
= 47.8 mL (1 mL = 0.001 L) = 0.0478 L
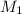 = 0.321 M,
= 0.321 M,
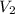 = 21.8 mL = 0.0218 L,
= 21.8 mL = 0.0218 L,
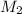 = 0.366 M
= 0.366 M
As concentration of a substance is the moles of solute divided by volume of solution.
Hence, concentration of aluminum cation is calculated as follows.
![[Al^(3+)] = (M_(1)V_(1) + M_(2)V_(2))/(V_(1) + V_(2))](https://img.qammunity.org/2022/formulas/chemistry/college/hc4rhtkdjd10jlxkj4s2qbf5r0b3d4ye9t.png)
Substitute the values into above formula as follows.
![[Al^(3+)] = (M_(1)V_(1) + M_(2)V_(2))/(V_(1) + V_(2))\\= (0.321 M * 0.0478 L + 0.366 M * 0.0218 L)/(0.0478 L + 0.0218 L)\\= (0.0153438 + 0.0079788)/(0.0696)\\= 0.335 M](https://img.qammunity.org/2022/formulas/chemistry/college/461k8jvl8vie33w4b4eud2dotjw6f8i37v.png)
Thus, we can conclude that the final concentration of aluminum cation is 0.335 M.