Answer:
4.11 moles of H₂ are in a 45.3 L container under the same conditions.
Step-by-step explanation:
Avogadro's law establishes the relationship between the amount of gas and its volume when the temperature and pressure are held constant.
This law says that the volume of a gas under conditions of constant temperature and pressure is directly proportional to the number of moles of gas. That is, if the amount of gas is increased, the volume will increase; whereas if the amount of gas is decreased, the volume decreases.
Avogadro's law is expressed mathematically as the quotient between the volume and the amount of gas equal to a constant:
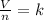
Being 1 the initial conditions and 2 the final conditions, the following is satisfied:
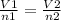
In this case:
- V1= 32.8 L
- n1= 2.98 moles
- V2= 45.3 L
- n2= ?
Replacing:
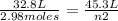
Solving:
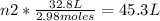
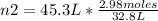
n2= 4.11 moles
4.11 moles of H₂ are in a 45.3 L container under the same conditions.