Answer: The value of
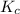 for this reaction is 250000.
for this reaction is 250000.
Step-by-step explanation:
The given equation is as follows.
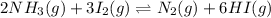
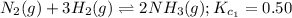 ... (1)
... (1)
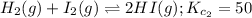 ... (2)
... (2)
To balance the atoms, multiply equation (2) by 3. Hence, the equation (2) can be re-written as follows.
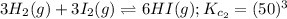 ... (3)
... (3)
Now, subtract equation (1) from equation (3). So, the equation formed will be as follows.
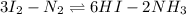
This equation can also be re-written as follows.
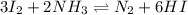
This equation is similar to the equilibrium equation given to us.
Therefore, during this subtraction the equation constants get divided as follows.
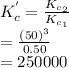
Thus, we can conclude that the value of
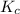 for this reaction is 250000.
for this reaction is 250000.