Answer:
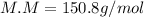
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly possible for us to set up the equation for the freezing point depression as shown below:
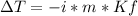
Thus, given Kf, i (equal to 1 because it is nonelectrolyte) and the freezing point depression, we can now calculate the molality of the solution:
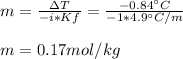
Next, we calculate the kilograms of solvent by dividing the 200 g by 1000 to get 0.200 kg and thus calculate the moles of the solute X:
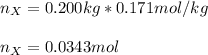
Finally, the molar mass by dividing the grams by moles:
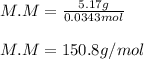
Regards!