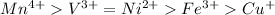Answer: The given transition metal ions in order of decreasing number of unpaired electrons are as follows.
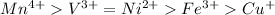
Step-by-step explanation:
In atomic orbitals, the distribution of electrons of an atom is called electronic configuration.
The electronic configuration in terms of noble gases for the given elements are as follows.
- Atomic number of Fe is 26.
![Fe^(3+) - [Ar] 3d^(5)](https://img.qammunity.org/2022/formulas/chemistry/college/pqbkwx4q5cc3nxcv60ivxvk0519grox33e.png)
So, there is only 1 unpaired electron present in
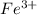 .
.
- Atomic number of Mn is 25.
![Mn^(4+) - [Ar]3d^(3)](https://img.qammunity.org/2022/formulas/chemistry/college/63c3yhee5rjbwp408hynszq3xxac2ofu8s.png)
So, there are only 3 unpaired electrons present in
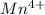 .
.
- Atomic number of V is 23.
![V^(3+) - [Ar] 3d^(2)](https://img.qammunity.org/2022/formulas/chemistry/college/kp9jorx63n76f065tjl456p7k696irspuf.png)
So, there are only 2 unpaired electrons present in
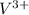 .
.
- Atomic number of Ni is 28.
![Ni^(2+) - [Ar] 3d^(8)](https://img.qammunity.org/2022/formulas/chemistry/college/pzvqa4wkzxmtme9ljuuiljymkf7wnfnqur.png)
So, there will be 2 unpaired electrons present in
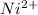 .
.
- Atomic number of Cu is 29.
![Cu^(+) - [Ar] 3d^(10)](https://img.qammunity.org/2022/formulas/chemistry/college/2w7kh7lryr15ypaj5ow2vvv4bgbcffiy3a.png)
So, there is no unpaired electron present in
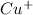 .
.
Therefore, given transition metal ions in order of decreasing number of unpaired electrons are as follows.
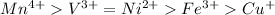
Thus, we can conclude that given transition metal ions in order of decreasing number of unpaired electrons are as follows.