Answer: The atomic radius of iron is 128 pm.
Step-by-step explanation:
To calculate the radius of the metal having FCC crystal lattice, the relationship between edge length and radius follows:
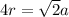
Where,
a = edge length = 362 pm
r = atomic radius of iron = ?
Plugging values in above equation, we get:
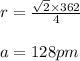
Hence, the atomic radius of iron is 128 pm.