Given :
A 3.82L balloon filled with gas is warmed from 204.9K to 304.8 K.
To Find :
The volume of the gas after it is heated.
Solution :
Since, their is no information about pressure in the question statement let us assume that pressure is constant.
Now, we know by ideal gas equation at constant pressure :
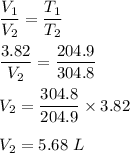
Hence, this is the required solution.