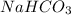Answer:
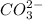
Step-by-step explanation:
Trioxocarbonate (iv) salts result from the reaction of trioxocarbonate(iv) acid with metals and metal oxides.
All sodium, potassium, and ammonium of trioxocarbonate(iv) salts appearas to be soluble, while all others are insoluble.
Apart from Na and K, all trioxocarbonate (iv) salts breakdown to release CO2 when heated.
The formula for Trioxocarbonate (iv) is:
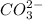
So, we can have Sodium trioxocarbonate as
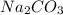 and sodium hydrogen trioxocarbonate (iv)
and sodium hydrogen trioxocarbonate (iv)