Answer:
The answer is C. 40.0 mL.
Step-by-step explanation:
To solve for the volume of KOH, start by using the formula
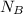
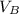 =
=
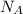
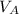 and label the information given in the question. The B in the formula stands for the base solution, and the A in the formula stands for the acid solution.
and label the information given in the question. The B in the formula stands for the base solution, and the A in the formula stands for the acid solution.
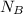 = 0.050 M KOH
= 0.050 M KOH
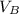 = ?
= ?
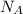 = 0.0100 M HNO3
= 0.0100 M HNO3
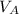 = 200. mL
= 200. mL
Next, use the formula
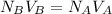 , and in order to find the volume for the base solution, the formula will have to be derived for
, and in order to find the volume for the base solution, the formula will have to be derived for
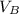 . The formula will now look like
. The formula will now look like
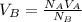 .
.
Then, plug in the information given in the question. The equation will look like
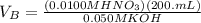 . Finally, solve the equation, and the answer will be 40.0 mL.
. Finally, solve the equation, and the answer will be 40.0 mL.