Given :
If a certain gas occupies a volume of 12 LL when the applied pressure is 6.0 atm.
To Find :
The pressure when the gas occupies a volume of 3.0 LL .
Solution :
Since, their is no information of temperature, let us assume it is constant throughout the process.
We know, by ideal gas equation at constant temperature :
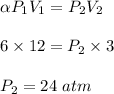
Therefore, the pressure when the gas occupies a volume of 3.0 LL is 24 atm.