Answer: The Ka expression for an aqueous solution of hypochlorous acid is
![K_(a) = ([H_(3)O^(+)][OCl^(-)])/([HClO])](https://img.qammunity.org/2022/formulas/chemistry/college/bec24su7h87yep8yu5ifsyuntebulyb5r8.png) .
.
Step-by-step explanation:
The chemical formula of hypochlorous acid is HClO. So, when it is added to water (solvent) then its dissociation is as follows.
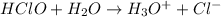
When we write the equilibrium constant for this reaction then it is called acid acid dissociated constant.
Hence, the expression for acid dissociation constant of this reaction is as follows.
![K_(a) = ([H_(3)O^(+)][OCl^(-)])/([HClO])](https://img.qammunity.org/2022/formulas/chemistry/college/bec24su7h87yep8yu5ifsyuntebulyb5r8.png)
Thus, we can conclude that the Ka expression for an aqueous solution of hypochlorous acid is
![K_(a) = ([H_(3)O^(+)][OCl^(-)])/([HClO])](https://img.qammunity.org/2022/formulas/chemistry/college/bec24su7h87yep8yu5ifsyuntebulyb5r8.png) .
.