Answer:
The molarity of ethanol is 5.8*10⁻³ M.
Step-by-step explanation:
Molar concentration is a measure of the concentration of a solute in a solution, be it some molecular, ionic or atomic species.
Molarity or Molar Concentration is the number of moles of solute that are dissolved in a certain volume. It is calculated by dividing the moles of the solute by the volume of the solution:
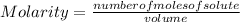
Molarity is expressed in units
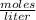 .
.
Being the molar mass of ethanol 46
 , then the number of moles that 40 grams of ethanol contain is calculated by:
, then the number of moles that 40 grams of ethanol contain is calculated by:
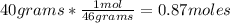
So, being:
- number of moles of solute= 0.87 moles
- volume 150 L
Replacing in the definition of molarity:
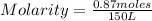
Solving:
Molarity= 5.8*10⁻³ M
The molarity of ethanol is 5.8*10⁻³ M.