The correct question is: 10 kg of R-134a at 300 kPa fills a rigid container whose volume is 14 L. Determine the temperature and total enthalpy in the container. The container is now heated until the pressure is 600 kPa. Determine the temperature and total enthalpy when the heating is completed.
Answer: The temperature is
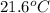 and total enthalpy when the heating is completed is 300 kJ.
and total enthalpy when the heating is completed is 300 kJ.
Step-by-step explanation:
Given: Mass = 10 kg
Volume = 14 L
Final pressure = 600 K
First, convert volume from L to
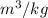 as follows.
as follows.
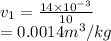
According to the R-134a tables at 300 kPa and
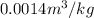 .
.
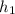 = 54.6 kJ/kg
= 54.6 kJ/kg
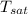 = 0.7 C
= 0.7 C
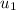 = 54.1 kJ/kg
= 54.1 kJ/kg
Now, at the state 2
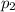 = 600 kPa and
= 600 kPa and
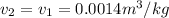
This means that the final temperature at state 2 is
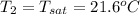
Hence, the change in enthalpy is calculated as follows.
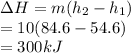
The first law is applied to transfer the heat transfer as follows.
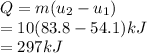
Thus, we can conclude that the temperature is
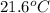 and total enthalpy when the heating is completed is 300 kJ.
and total enthalpy when the heating is completed is 300 kJ.