Answer:
28500 years
Step-by-step explanation:
Applying,
A = A'(
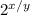 )............... Equation 1
)............... Equation 1
Where A = Original mass of Carbon-14, A' = Final mass of carbon-14 after decaying, x = total time, y = half-life.
From the question,
Given: A = 1 g, A' = 31.3 mg = 0.0313 g, y = 5700 years.
Substitute these values into equation 1
1 = 0.0313(
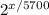 )
)
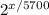 = 1/0.0313
= 1/0.0313
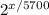 = 31.95
= 31.95
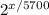 ≈ 32
≈ 32
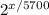 ≈ 2⁵
≈ 2⁵
Equating the base and solve for x
x/5700 ≈ 5
x ≈ 5×5700
x ≈ 28500 years