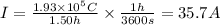Answer:
35.7 A
Step-by-step explanation:
Let's consider the reduction half-reaction of Al³⁺.
Al³⁺ + 3 e⁻ ⇒ Al
We will calculate the charge required to produce 18.0 g of Al using the following conversion factors.
- 1 mole of Al has a mass of 27.0 g
- 1 mole of Al is formed upon the circulation of 3 moles of electrons
- 1 mole of electrons has a charge of 96486 C (Faraday's constant)
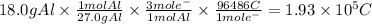
1.93 × 10⁵ C circulate in 1.50 hours. The intensity is: