Answer:
There are 0.301 moles of N2 gas in 6.75 liters of N2 gas.
Step-by-step explanation:
Given,
Volume of N2 gas = 6.75 L
We know,
At STP,
Number of moles =
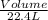 [Here 22.4 liter is the molar volume of any gas at STP]
[Here 22.4 liter is the molar volume of any gas at STP]
=
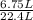
=
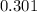
∴There are 0.301 moles of N2 gas in 6.75 liters of N2 gas.