Answer:

Step-by-step explanation:
Molarity is a measure of concentration in moles per liter, so the formula is:
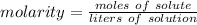
This solution has 9.0 moles of solute and 462 milliliters of solution. We must convert milliliters to liters. Remember that 1 liter contains 1000 milliliters.
Create a ratio.
Multiply by the value we are converting: 462 milliliters
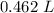
Now we know both values and we can solve for the molarity.
- moles of solute= 9.0 moles
- liters of solution = 0.462 L
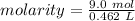
Divide.
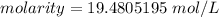
The original measurements of moles and milliliters have 2 and 3 significant figures respectively. We have to round our answer to the least number of sig figs, which is 2 in this case.
For the number we found, that is the ones place. The 4 in the tenths place (19.4805195) tells us to leave the 9 in the ones place.
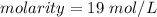
1 mole per liter is equal to 1 molar or M, so our answer is equal to 19 M.
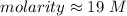
The molarity of the solution is approximately 19 M.