Answer:
NaOH is the limiting reactant.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly necessary to write out the chemical reaction between NaOH and HCl:

Thus, since they react in a 1:1 mole ratio; we can now calculate the moles of each substance by using their volumes and molarities:
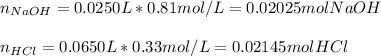
Now, since NaOH is in a fewer proportion, we infer just 0.02025 moles of HCl are consumed so that 0.0012 moles of this acid remain unreacted; in such a way, we infer that the NaOH is the limiting reactant for this reaction.
Regards!