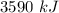Answer:
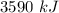
Step-by-step explanation:
Given
Paddle wheel work is
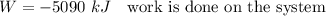
Heat transfer from the tank is
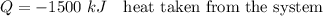
From the first law of thermodynamics
Change in the internal energy of the system is equal to the difference of heat and work .
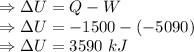
Therefore, the change in internal energy is