Answer:
The entropy change in the environment is 3.62x10²⁶.
Step-by-step explanation:
The entropy change can be calculated using the following equation:
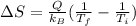
Where:
Q: is the energy transferred = 5.0 MJ
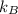 : is the Boltzmann constant = 1.38x10⁻²³ J/K
: is the Boltzmann constant = 1.38x10⁻²³ J/K
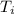 : is the initial temperature = 1000 K
: is the initial temperature = 1000 K
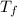 : is the final temperature = 500 K
: is the final temperature = 500 K
Hence, the entropy change is:
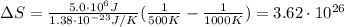
Therefore, the entropy change in the environment is 3.62x10²⁶.
I hope it helps you!