Answer:
0.5447
Step-by-step explanation:
The atomic packing fraction factor is given by
APF = Volume of sphere/ Volume of unit cell
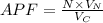
The atomic radius = 0.135 nm
The Density of the metal = 3.55 g/cm^3
To calculate the number of atoms
= desnsity/(atomic radius×Avagadro's number)
Putting values and solving we get
the number of atoms = 4
Now,
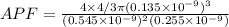
Solving we get
APF = 0.54427