Answer:
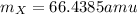
Step-by-step explanation:
Hello there!
In this case, according to the given information for this problem, it turns out possible for us to calculate the average atomic mass of the element by multiplying the mass of each isotope by its percent abundance and then add up the results, just as shown below:

Regards!