Answer:
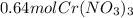
Step-by-step explanation:
Hello there!
In this case, according to the given chemical equation, it turns out possible for us to realize there is a 4:3 mole ratio of chromium(III) nitrate to lead(IV) nitrate, and therefore, we can calculate the moles of the former by applying the shown below stoichiometry setup:
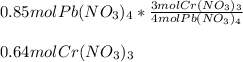
Regards!