Answer:
There are 122.1982401 moles of H2O in
 molecules of H2O
molecules of H2O
Step-by-step explanation:
We know,
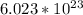 molecules of H2O contains 1 mole H2O
molecules of H2O contains 1 mole H2O
1 molecules of H2O contains
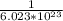 mole H2O
mole H2O
∴
 molecules of H2O contains
molecules of H2O contains
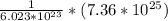 moles of H2O
moles of H2O
= 122.1982401 moles of H2O
∴ There are 122.1982401 moles of H2O in
 molecules of H2O
molecules of H2O