To Find :
The number of molecules of carbon tetrachloride in 0.32 mol of
carbon tetrachloride.
Solution :
We know, 1 mole of any element/compound contains
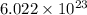 number of atoms/molecules.
number of atoms/molecules.
So, Number of molecules in 0.32 mol are :
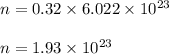
Therefore, number of molecules of carbon tetrachloride in 0.32 mol of
carbon tetrachloride are
 .
.