Answer: Moles are found in a 350.mL solution of 13.5M
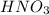 are 4.725 mol.
are 4.725 mol.
Step-by-step explanation:
Given: Volume = 350 mL (1 mL = 0.001 L) = 0.350 L
Molarity = 13.5 M
Molarity is the number of moles of a substance present in a liter of solution.
Therefore, moles of
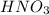 present in the given solution are calculated as follows.
present in the given solution are calculated as follows.
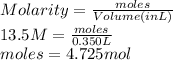
Thus, we can conclude that moles found in a 350.mL solution of 13.5M
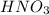 are 4.725 mol.
are 4.725 mol.