Answer: It is true for the process used to achieve the required dilution that volume of the solvent used is less than 2 liters.
Step-by-step explanation:
Given:
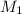 = 10 M,
= 10 M,
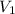 = ?
= ?
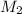 = 1 M,
= 1 M,
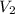 = 2 L
= 2 L
The volume of stock solution is calculated as follows.
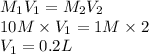
A solution which contains small amount of solute is called a dilute solution. Whereas a solution which contains more amount of solute is called a concentrated solution.
The stock solution is used for dilution and hence, it acts as a solvent. Its calculated volume is 0.2 L.
This means that volume of the solvent used is less than 2 liters.
Thus, we can conclude that it is true for the process used to achieve the required dilution that volume of the solvent used is less than 2 liters.