Answer: The pH of the solution is 2.74.
Step-by-step explanation:
To calculate the pH of the acidic buffer, the equation for Henderson-Hasselbalch is used:
![pH=pK_a+\log (\frac{\text{[conjugate base]}}{\text{[acid]}})](https://img.qammunity.org/2022/formulas/chemistry/college/6wa5iv167b7cqj1hpm3q2pb1ztyblc1aib.png) .......(1)
.......(1)
The power of the acid dissociation constant is the negative logarithm of the acid dissociation constant. The equation for it is:
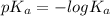 ......(2)
......(2)
The chemical equation for the reaction of acetic acid and NaOH follows:
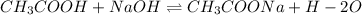
Given values:
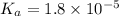
Putting values in equation 2:
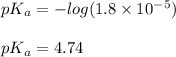
We are given:
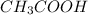 = 0.1 M
= 0.1 M
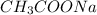 = 0.001 M
= 0.001 M
Plugging values in equation 1:
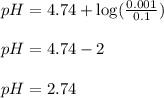
Hence, the pH of the solution is 2.74.