Answer: 400 g of solute is present in the solution
Step-by-step explanation:
A solution consists of solute and solvent. A solute is defined as the component that is present in a smaller proportion and solvent is defined as the component that is present in a larger proportion.
Saturation point of a solution is defined as the point after which the solution becomes saturated. This simply means no more solute can be added to the solution.
Mass of solvent = 800 g
At saturation point, 500 g of solvent can dissolve 250 g of solute
Applying unitary method:
800 g of solvent will dissolve =
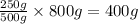 of solute
of solute
Hence, 400 g of solute is present in the solution.