Answer:
Q = 669.44 J
Step-by-step explanation:
Given that,
Mass of water, m = 32 g
The temperature change from 25.0°C to 20.0°C.
We need to find the amount of heat energy transferred. Let it is Q. We know that,
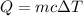
Where
c is the specific heat of water
Put all the values,
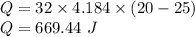
So, 669.44 J of heat energy is transferred from the water.