Answer:
a) 3.9 x 10⁻⁵ kg
Step-by-step explanation:
The amount of mass required to produce the energy can be given by Einstein's formula:
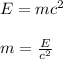
where,
m = mass required = ?
E = Energy produced = 3.5 x 10¹² J
c = speed of light = 3 x 10⁸ m/s
Therefore,
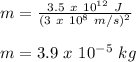
Hence, the correct option is:
a) 3.9 x 10⁻⁵ kg