The question is incomplete. The complete question is :
Iron β is a solid phase of iron still unknown to science. The only difference between it and ordinary iron is that Iron β forms a crystal with an fcc unit cell and a lattice constant, a = 0.352 nm. Calculate the density of Iron β.
Solution :
The density is given by :
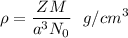 ..................(i)
..................(i)
Here, Z = number of atoms in a unit cell
M = atomic mass
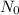 = Avogadro's number =
= Avogadro's number =
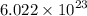
a = edge length or the lattice constant
Now for FCC lattice, the number of atoms in a unit cell is 4.
So, Z = 4
Atomic mass of iron, M = 55.84 g/ mole
Given a = 0.352 nm =
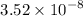 cm
cm
From (i),
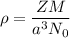
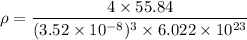
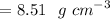
Therefore, the density of Iron β is
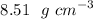 .
.