Step-by-step explanation:
Given that,
Initial volume = 60dm³
Initial pressure = 30 kPa
We need to find the new volume when pressure changes to 40 kPa (say).
We know that the mathematical relation between volume and pressure is given by :
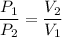
Put all the values,
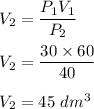
So, when pressure of the gas increases, its volume get decreased.